Fluorescence microscopy is a cornerstone technique in cell biology. Fluorescent resonant energy transfer (FRET) microscopy is an advanced imaging tool to study conformational changes in proteins and protein-protein interactions. FRET is a phenomenon that occurs when two different fluorophores are in close proximity (<10 nm) to each other, where the emission spectrum of one (the donor) overlaps with the excitation spectrum of the other (the acceptor). The efficiency of FRET is highly dependent on the distance between the donor and acceptor fluorophore, and therefore can be used to precisely measure intra- and intermolecular interactions. Using fluorescence lifetime microscopy (FLIM) we can measure FRET by measuring a decrease in fluorescence lifetime of the donor fluorophore.
In this project you will use FRET/FLIM to investigate protein-protein interactions and the role of dimerisation of the androgen receptor (AR). This nuclear receptor is present in the cell in different conformations and dimerizes upon activation by binding to testosterone. Using fluorescently labeled androgen receptors that are fluorescently tagged at both the N- and the C- terminus by a donor and acceptor fluorophore respectively, we monitor the conformation using FRET/FLIM. We hypothesize that AR is in a closed conformation (where the N- and C-terminus are close together and FRET will occur) when it is not bound to DNA and opening up when it is binding to DNA and activating transcription (no FRET, because N- and C-termini are far away from each other.
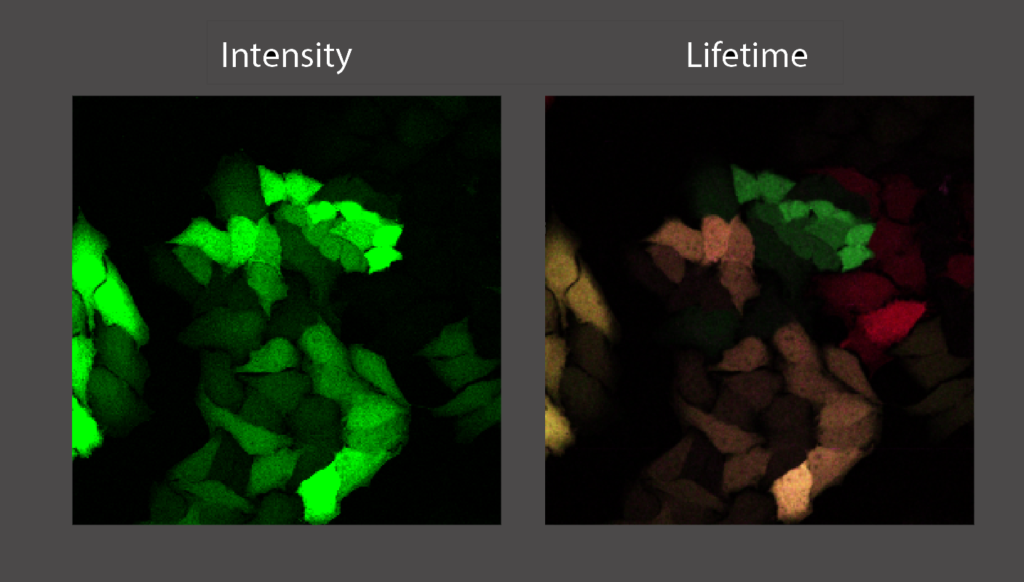
This project is focused on advanced microscopy but will also involve cell culture, as well as the design and cloning of fusion proteins. The results of this study will help us to better understand the function of the androgen receptor. This will lead to further insight about AR and its role in prostate cancer, the most prominent form of cancer in males.
Contact Information:
Erasmus Optical Imaging Center
www.erasmusoic.nl
Johan Slotman (j.slotman [a] erasmusmc.nl)
Tel: 010-7037644

